
Because it's a nonmetal, arsenic contains a high density, moderate thermal conduction, and a restricted ability to conduct electricity. Arsenic may be an extremely toxic metalloid. The electron configuration of As is 3d 10 4s 2 4p 3. Group 15 Element’s Electron Configuration The main source of phosphorus compounds is phosphorus rocks. Phosphorus is the eleventh most abundant component, making up 0.11% of the Earth's crust. The most common oxidation state of phosphorus is -3. The electron configuration of P is 3s 2 3p 3. The common oxidation states of (atomic number 7) N are +5, +3, and -3. When compared with the rest of group fifteen, N has the highest electronegativity which makes it the most nonmetallic of the group. It is a non-metallic component and has no taste and no colour. The electronic configuration of N is 1s 2 2s 2 2p 3. The filling of electrons into the orbitals of different atoms takes place according to the Aufbau principle which is based on Pauli’s exclusion principle, Hund’s rule of maximum multiplicity, and the relative energies of the orbitals.Įlectronic Configuration of Group 15 Elements Nitrogen: (Atomic no. Rules to determine the electronic configuration for any element are listed below: Rules to Write the Electronic Configuration Eventually, the superscript indicates how many electrons are in the orbital.
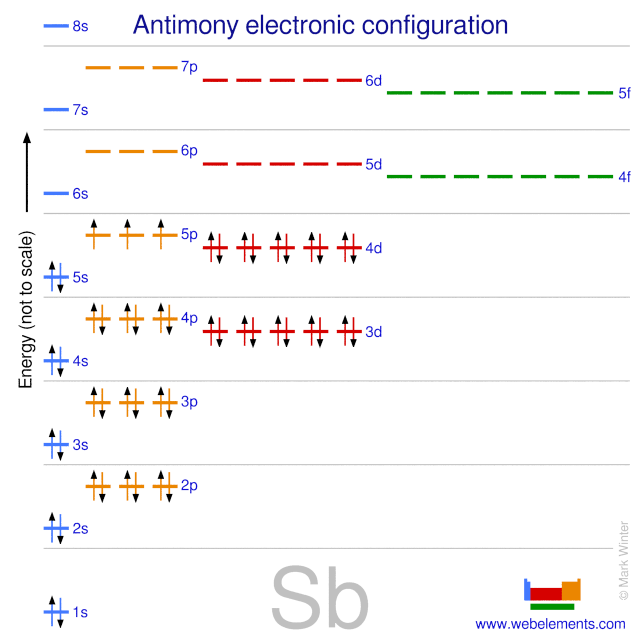
The symbols used for writing the electron configuration begin with the shell number (n) followed by the type of orbital. Electronic configuration, also referred to as electronic structure or electron configuration, is the arrangement of electrons in orbitals around an atomic nucleus.


 0 kommentar(er)
0 kommentar(er)
